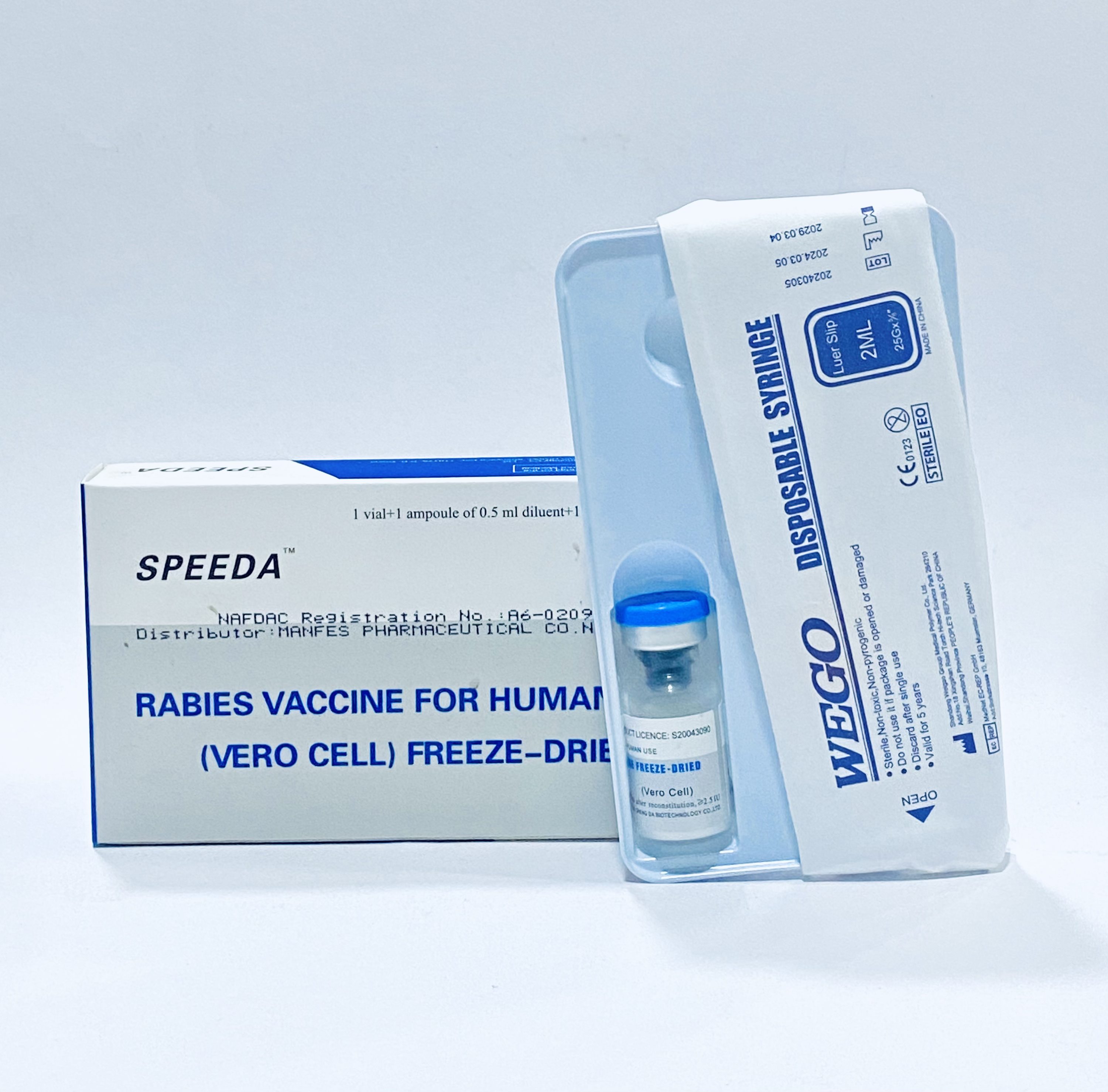
Abhayrab Rabies Vaccine is a purified Vero cell rabies vaccine for human use. This inactivated vaccine provides active immunization against rabies virus and can be administered intramuscularly or intradermally for both pre-exposure prophylaxis and post-exposure treatment.
Uses
Abhayrab Rabies Vaccine is used for pre-exposure prophylaxis in high-risk individuals like veterinarians, animal handlers, and travelers to rabies-endemic areas. It’s also used for post-exposure prophylaxis following animal bites or scratches from potentially rabid animals. The vaccine is essential for preventing rabies infection in exposed individuals.
Benefits
- Provides effective protection against rabies virus
- Suitable for both pre-exposure and post-exposure prophylaxis
- Can be administered through intramuscular or intradermal routes
- Demonstrates high immunogenicity and safety profile
- Reduces need for rabies immunoglobulin in some cases
- Provides long-lasting immunity when used properly
- Safe for use in all age groups including infants
- Compatible with other vaccines when given simultaneously
- Purified cell culture vaccine with minimal side effects
How It Works
Abhayrab Rabies Vaccine contains inactivated rabies virus grown in Vero cells that stimulates the immune system to produce antibodies against rabies. These antibodies provide protection against future rabies virus exposure. The vaccine induces both humoral and cellular immune responses, creating immunological memory for long-term protection.
Dosage
| Route |
Pre-exposure Schedule |
Post-exposure Schedule |
| Intramuscular |
Days 0, 7, 21 or 28 |
Days 0, 3, 7, 14, 28 |
| Intradermal |
Days 0, 7, 21 or 28 |
Days 0, 3, 7, 14, 28 |
Administer 1ml intramuscularly in deltoid muscle or 0.1ml intradermally. Complete the full schedule for optimal protection.
Duration of Action
Abhayrab Rabies Vaccine begins producing antibodies within 7-14 days of first dose. Protective antibody levels typically develop after the second dose in pre-exposure schedules. Post-exposure protection develops progressively with each dose. Immunity can last 2-3 years after primary series, with boosters recommended based on antibody levels.
Side Effects
Common local reactions include pain, redness, and swelling at injection site. Systemic effects may include mild fever, headache, muscle aches, and fatigue. These reactions are typically mild and resolve within 24-48 hours. Serious allergic reactions are rare but require immediate medical attention.
Warning
Do not use in individuals with severe illness or known hypersensitivity to vaccine components. Immunocompromised patients may have reduced response and require antibody level monitoring. Never administer in the gluteal region as this may result in vaccine failure. Seek immediate medical care for severe allergic reactions.
Pregnancy and Breastfeeding
Abhayrab Rabies Vaccine can be used during pregnancy when risk of rabies exposure exists, as rabies is fatal if untreated. No adverse effects have been reported during pregnancy. The vaccine is considered safe during breastfeeding as it does not affect milk production or infant safety.
Interaction
- Generally compatible with other vaccines when given at different sites
- May have reduced effectiveness in immunocompromised patients
- Concurrent use with immunosuppressive drugs may reduce immune response
- Can be given with rabies immunoglobulin but at different injection sites
- No known interactions with routine medications
Precaution
Store vaccine at 2-8°C and protect from light. Do not freeze or shake vigorously. Use within recommended time after opening. Ensure proper sterile technique during administration. Monitor for allergic reactions for 15-20 minutes after vaccination. Document vaccination dates and maintain records.
Important Information
Abhayrab Rabies Vaccine does not provide immediate protection and must be combined with wound care and rabies immunoglobulin when indicated for post-exposure treatment. Complete the full vaccination schedule even if no symptoms develop. Pre-exposure vaccination does not eliminate need for post-exposure treatment but may reduce number of doses required. Consult healthcare provider for individual risk assessment and vaccination recommendations.




























Reviews
There are no reviews yet.