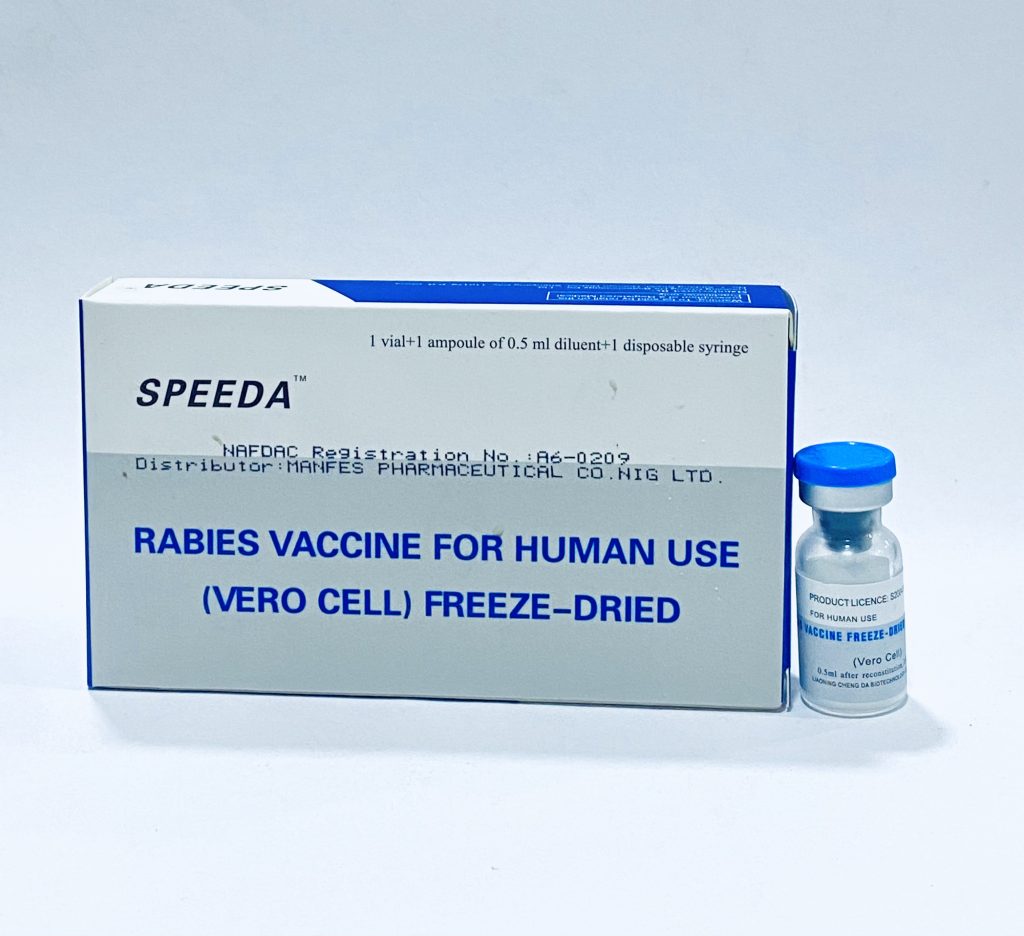
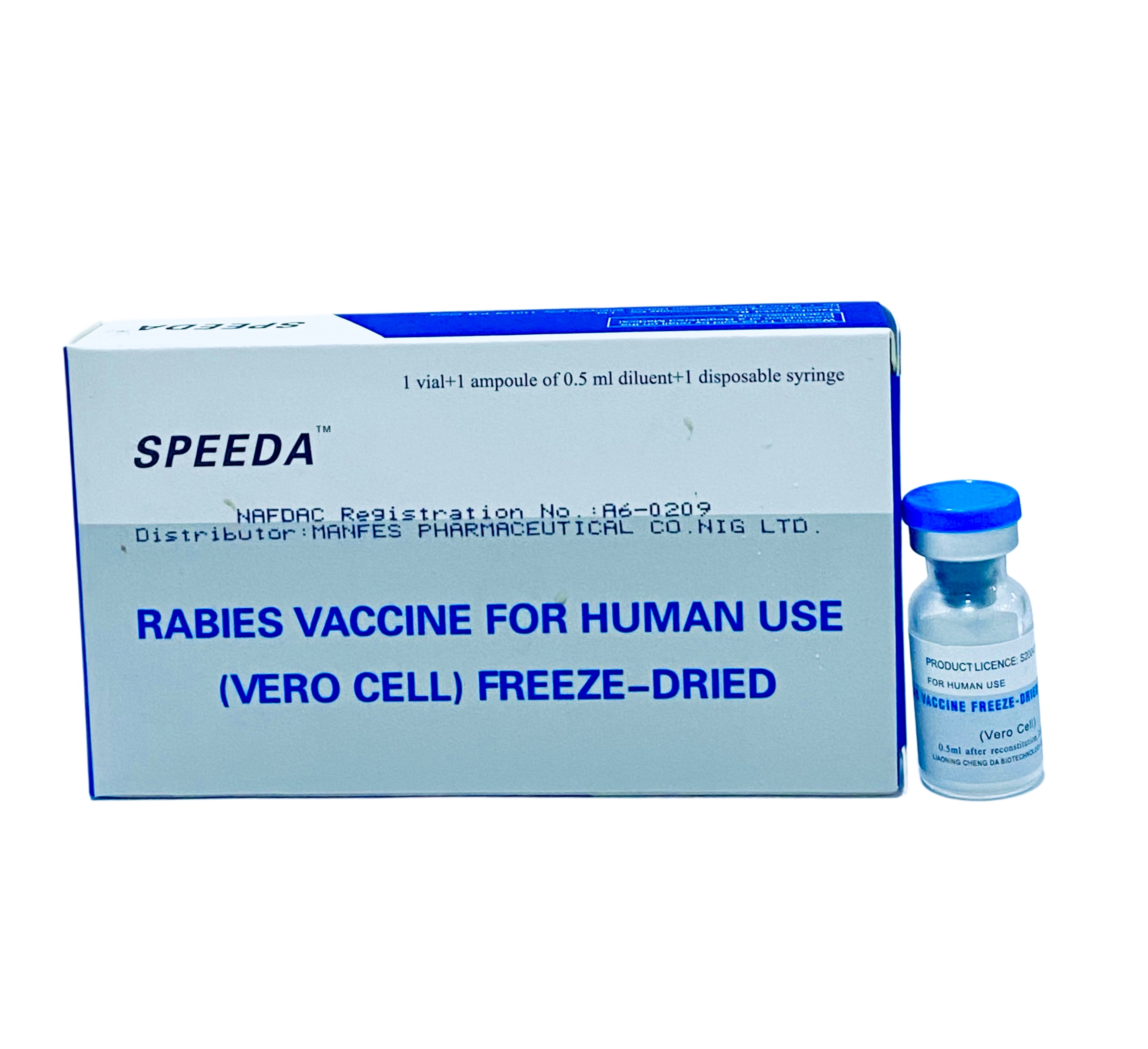
Speeda Anti-Rabies Vaccine is a freeze-dried, Vero cell-derived vaccine for human use against rabies. This purified inactivated vaccine provides immunization against the rabies virus following animal bites or high-risk exposures, and can be used for both pre-exposure prophylaxis and post-exposure treatment.
Uses
Speeda Anti-Rabies Vaccine is used for pre-exposure prophylaxis in high-risk individuals (veterinarians, laboratory workers, animal handlers) and for post-exposure treatment following potential rabies exposure through animal bites, scratches, or contact with saliva. It stimulates the immune system to produce protective antibodies against the rabies virus.
Benefits
- Provides effective protection against potentially fatal rabies infection
- Suitable for both pre-exposure prophylaxis and post-exposure treatment
- Produces reliable antibody response with proper administration
- Manufactured using Vero cell technology for consistent quality
- Contains no animal proteins, reducing risk of allergic reactions
- Freeze-dried formulation offers extended shelf life
- Compatible with rabies immunoglobulin when needed
- Established safety profile across different age groups
- Meets WHO standards for rabies vaccine potency
How It Works
Speeda contains inactivated rabies virus antigens that stimulate the immune system to produce protective antibodies. When administered through the intramuscular route, it triggers specific B-cells to generate antibodies that neutralize the rabies virus before it can reach the nervous system, preventing the development of clinical rabies.
Dosage
| Use Case | Injection Schedule | Route |
|---|---|---|
| Pre-exposure | Days 0, 7, 28 (3 doses) | Intramuscular |
| Post-exposure | Days 0, 3, 7, 14, 28 (5 doses) | Intramuscular |
Reconstitute the freeze-dried vaccine with provided diluent immediately before use. Administer intramuscularly in the deltoid region for adults or anterolateral thigh for children.
Duration of Action
Speeda Anti-Rabies Vaccine begins generating antibodies within 7-10 days after the first dose. Following complete pre-exposure prophylaxis, protective antibody levels typically last 2-3 years. Post-exposure treatment provides immediate protection when completed correctly, with booster doses recommended for continued immunity in high-risk individuals.
Side Effects
Common side effects include injection site reactions (pain, redness, swelling), mild fever, headache, and fatigue. These typically resolve within 1-2 days. Less common effects include nausea, muscle aches, and dizziness. Serious allergic reactions are rare but require immediate medical attention.
Warning
Rabies is nearly always fatal once symptoms develop. Do not delay post-exposure treatment while awaiting test results from animal. No contraindication exists for post-exposure vaccination. Maintain cold chain during vaccine storage. Incomplete vaccination schedules may not provide adequate protection. Seek immediate medical care for any suspected rabies exposure.
Pregnancy and Breastfeeding
Speeda Anti-Rabies Vaccine can be administered during pregnancy and breastfeeding when indicated. Rabies exposure poses a greater risk than the vaccine to both mother and fetus. No adverse effects on pregnancy outcomes or breastfed infants have been documented from rabies vaccination.
Interaction
- No significant interaction with common medications
- Can be administered concurrently with other vaccines at different sites
- May have reduced effectiveness in immunocompromised individuals
- Compatible with rabies immunoglobulin when given at separate sites
- No known interference with laboratory tests
Precaution
Individuals with history of severe allergic reactions to previous rabies vaccines require careful monitoring. Those with moderate to severe acute illness may defer pre-exposure vaccination until recovery, but post-exposure treatment should not be delayed. Immunocompromised persons may require antibody testing to confirm protective response.
Important Information
Speeda Anti-Rabies Vaccine is not a substitute for proper wound care, which should include thorough washing with soap and water. For post-exposure treatment, rabies immunoglobulin may be recommended in addition to the vaccine. Keep vaccination records for future reference. Maintain the cold chain (2-8°C) for vaccine storage. Do not freeze the vaccine or diluent. Reconstituted vaccine should be used immediately.
Product Image Highlights
Customer questions & answers
There are no questions yet. Be the first to ask a question about this product.
A Special Pick for You
-
 Rated 0 out of 5
Rated 0 out of 5Midlife Meropenem Injection is used to treat serious bacterial infections,...
-
 Rated 0 out of 5
Rated 0 out of 5Actrapid is used for the treatment of diabetes mellitus in...
-
 Rated 0 out of 5
Rated 0 out of 5Taxiject Injection 1.0g is used to treat a variety of...
-
 Rated 0 out of 5
Rated 0 out of 5Gestone Injection is used for luteal phase support in Assisted...










Reviews
There are no reviews yet.