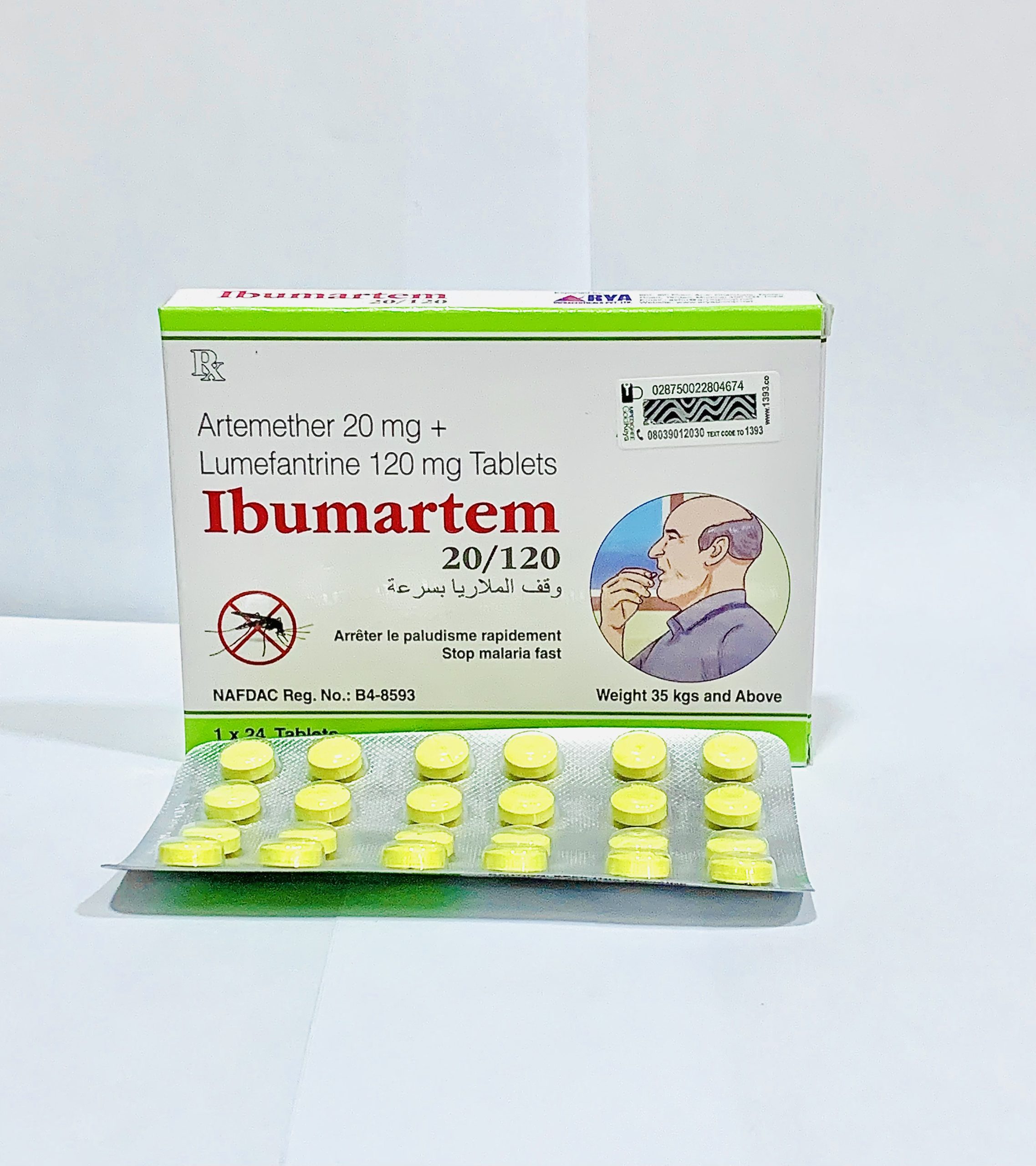
Ibumartem 20/120mg Tablet is an antimalarial medication combining Artemether (20mg) and Lumefantrine (120mg). This dual-action therapy effectively treats acute uncomplicated malaria caused by Plasmodium falciparum, including strains resistant to other antimalarial drugs.
Uses
Ibumartem 20/120mg Tablet treats acute uncomplicated malaria caused by Plasmodium falciparum. It’s particularly valuable against strains resistant to other antimalarials like chloroquine, amodiaquine, and sulfadoxine-pyrimethamine.
Benefits
- Highly effective against acute uncomplicated P. falciparum malaria
- Eliminates strains resistant to other antimalarial medications
- Well-tolerated with favorable safety profile
- Prevents recurrence of infection
- Rapidly reduces parasite load
- Alleviates malaria symptoms quickly
- Convenient dosing schedule
- Suitable for various patient populations
- Established efficacy in endemic regions
How It Works
Artemether and Lumefantrine work synergistically to eliminate malaria parasites at different lifecycle stages. Artemether rapidly clears blood parasites through free radical generation, while Lumefantrine prevents recrudescence by eliminating residual parasites, ensuring complete clearance of the infection.
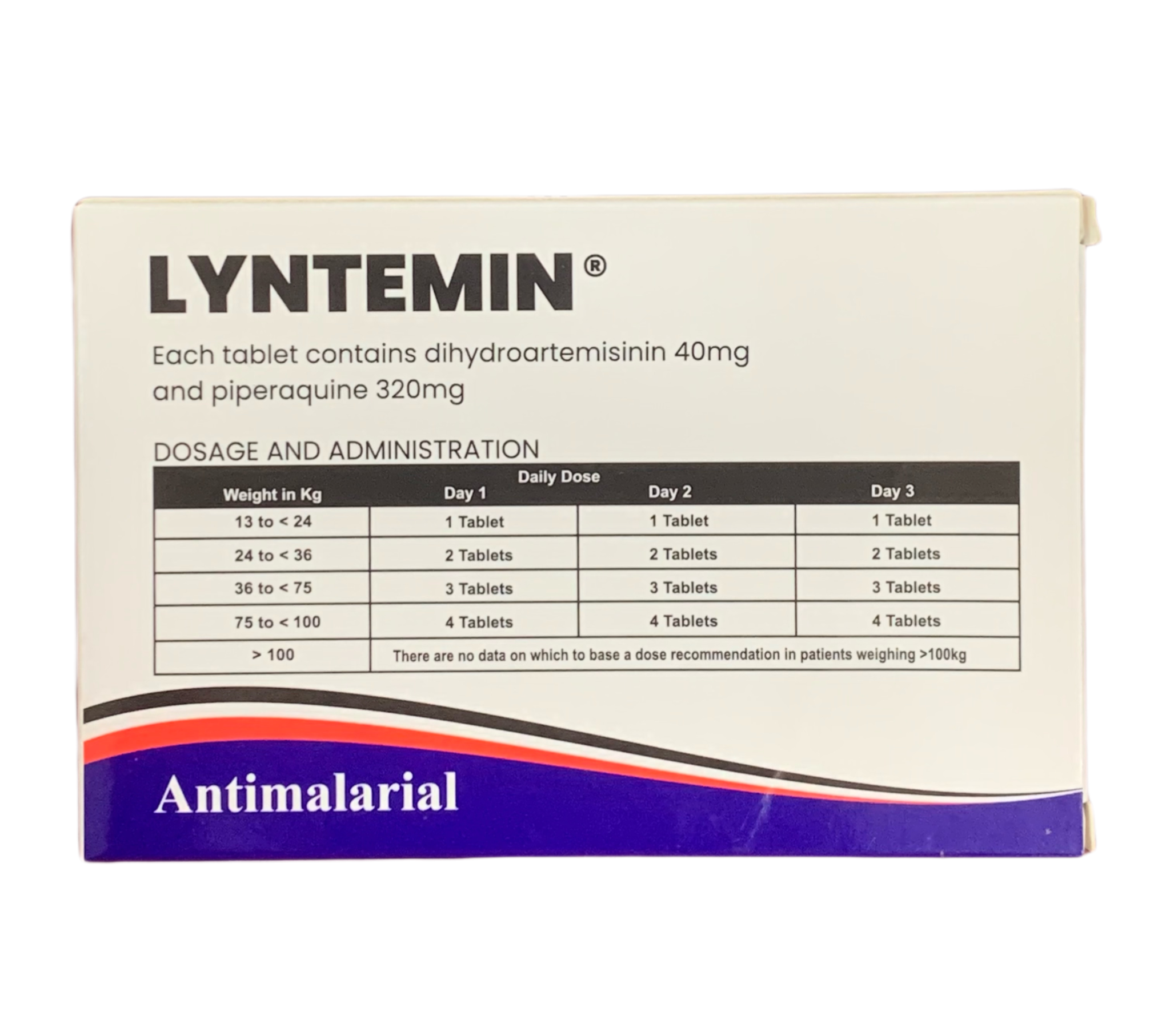
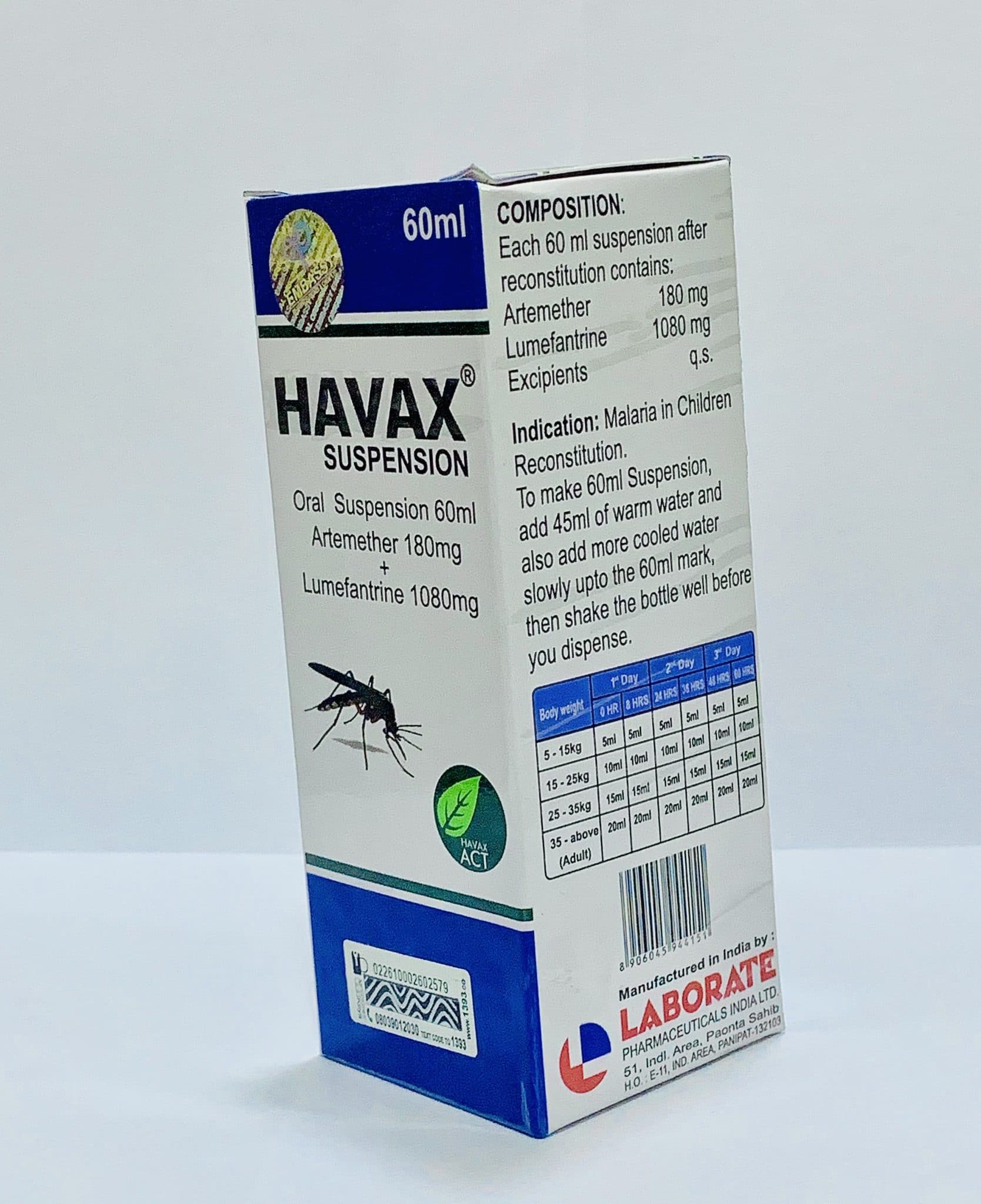
Dosage
| Patient Weight | Dosing Schedule | Total Course |
|---|---|---|
| Adults & ≥35kg | 4 tablets twice daily (morning & evening) | 24 tablets over 3 days |
| 25kg to <35kg | 3 tablets twice daily (morning & evening) | 18 tablets over 3 days |
| 15kg to <25kg | 2 tablets twice daily (morning & evening) | 12 tablets over 3 days |
Take Ibumartem with food or milk to improve absorption. Space doses approximately 12 hours apart.
Duration of Action
Ibumartem begins working within hours of the first dose, with most patients experiencing symptom improvement within 24-48 hours. Artemether provides rapid parasite clearance while Lumefantrine’s longer half-life prevents recurrence for approximately 4 weeks following treatment completion.
Side Effects
Common side effects include headache, dizziness, nausea, vomiting, abdominal pain, fatigue, decreased appetite, and muscle/joint pain. Most side effects are mild to moderate and resolve without intervention as treatment progresses.
Warning
Not for use in severe malaria cases requiring parenteral treatment. Avoid in patients with known hypersensitivity to ingredients. Use caution in patients with cardiac conditions as Ibumartem may prolong QT interval. Complete the full treatment course even if symptoms improve. Seek medical attention if symptoms worsen.
Pregnancy and Breastfeeding
Ibumartem can be used in the second and third trimesters if benefits outweigh risks. First trimester use only when no alternatives exist and malaria presents life-threatening risk. Limited data exists on breastfeeding safety, though benefits typically outweigh risks in malaria-endemic regions.
Interaction
- May interact with medications that prolong QT interval
- Effectiveness reduced by CYP3A4 inducers (rifampicin, carbamazepine)
- Concentration increased by CYP3A4 inhibitors (ketoconazole)
- Potential interactions with antiretroviral medications
- Grapefruit juice may increase drug levels
Precaution
Use with caution in patients with liver or kidney impairment. Not recommended for prophylaxis. Avoid in patients with family history of sudden death or congenital QT prolongation. If vomiting occurs within 1 hour of dose, repeat the dose. Monitor patients with electrolyte abnormalities closely.
Important Information
Take with fatty food or milk to maximize absorption. Complete the full 3-day treatment course even if feeling better. Continue protective measures against mosquito bites to prevent reinfection. Store below 30°C in original packaging protected from light and moisture. Consult healthcare provider if fever persists after treatment completion.









Reviews
There are no reviews yet.